자료실
Precision laser marking for medical applications
본문
15-12-28 14:58
 Precision laser marking for medical
applications Precision laser marking for medical
applications |
| Ultraviolet DPSS
lasers work with wider range of materials JORG HELLER Marking is critical for medical devices and pharmaceuticals, both to enable product tracking and identification and to combat counterfeiting. Traditionally, these marks have been printed with ink or, more recently, produced with infrared (IR) lasers. However, both of these approaches have disadvantages. Ultraviolet (UV) diode-pumped solid-state (DPSS) lasers overcome virtually all the drawbacks of these other technologies, but have rarely been utilized in the past because of their cost. But significant reductions in capital expenditure (capex) and cost of ownership now make UV laser marking very attractive for many medical applications. This article reviews its characteristics and presents some applications results for medical and pharmaceutical uses. Medical marking The requirements for marking medical products are somewhat unique compared to other industries. Pills are ingested, and many other medical products (tubing, stents, etc.) are brought into either external or internal contact with patients. So, it is often essential that the mark itself is not a source of contamination, or does not contain chemicals that might cause an allergic reaction. Furthermore, it is frequently desirable that the marking process leave the marked surface smooth, either to avoid tissue damage or to avoid having the mark become a site for bacterial growth |
 |
| Another frequent
requirement of medical marks is the inclusion of lot numbers, serial numbers, or
other identifying information that enables the determination of where and when a
specific product was manufactured. Then, if units are subsequently found to be
somehow defective, users can readily identify if they possess products made at
the same time and place that might exhibit a similar problem. Batch and origin
marking also helps to address a growing problem in the pharmaceutical and
medical industries, namely counterfeiting and "gray market" products. Sometimes,
this counterfeiting involves nothing more than simply peeling a label off a
bottle and applying a new one. But, increasingly, western countries are being
flooded with counterfeit pharmaceuticals (usually produced in Asia) that appear
identical to the real version, but may not contain the correct dosages or meet
requisite quality control standards. As a result, the ideal mark for medical
applications is indelible, easy to read, difficult to copy or alter, contains
unique serialization information, and doesn't change product functionality in
any way. Traditional marking methods The dominant method for marking pharmaceuticals, medical devices, and their associated packaging has long been ink printing (inkjet or pad printing). Pills are usually imprinted using an offset rotogravure method. Printing is attractive to manufacturers because the capital equipment cost is relatively low. However, the ongoing cost of consumables (ink) is often substantial. For medical applications, the main drawback of printing is that it is often easy to remove or alter printed marks (especially if they're on a paper label). This means that marks can become difficult to read after shipping, handling, or storage, and also permits purposeful counterfeiting. Print quality is also limited, and this represents a problem as manufacturers try to squeeze ever more information, including 2D barcodes, into a small space. For pill printing (in particular), offset techniques, which apply pressure to the product, are difficult to implement with increasingly popular "softgel" capsules. |
 |
| While the inks employed
for printing pharmaceutical and medical equipment are non-toxic, the printing
equipment itself is often "dirty," utilizing lubricants and solvents that can
become airborne and contaminate printed products. Also, printing equipment is
often mechanically complex, requiring downtime for cleaning and maintenance.
Laser marking is a non-contact method that avoids the problems of contamination
and eliminates the cost of consumables. Furthermore, laser marking generally
supports the creation of high-contrast, high-resolution marks that can be made
very small and readily be applied to curved or contoured surfaces. Laser marking
usually employs either CO2 or solid-state lasers operating in the IR. The
marking process itself is a thermal interaction; material is heated until it
bleaches, carbonizes, or ablates to produce a color contrast. Nearly all
plastics directly absorb the far-IR CO2 output?absorptive additives are
sometimes used to facilitate this process with a near-IR, solid-state laser.
However, this heating can alter the chemical structure of the material in the
heat-affected zone (HAZ), and also produces some surface relief. This texture
can offer a place for bacteria to settle and grow, and may be difficult to
clean. UV laser marking The laser/material interaction is fundamentally different in the UV than in the IR. Specifically, the UV (355 nm) output of a frequency-tripled DPSS laser is typically absorbed more strongly than longer wavelengths. It then undergoes a cold photochemical (rather than photothermal) interaction with any fillers or pigments within the plastic. For most plastics that appear white, this pigment is titanium dioxide (TiO₂), which strongly absorbs the UV light and then undergoes a change in crystalline structure. This renders the substance dark, producing a smooth, highly legible mark within the bulk material rather than at the surface. |
 |
| Because the mark is
actually subsurface, it doesn't provide a possible home for bacteria, and it is
nearly impossible to alter or deface without destroying the material itself.
Furthermore, since this is a cold process, there is essentially no HAZ or
changes to the surrounding material. And, the higher absorption in the UV means
that material can be processed with lower laser power (or pulse energy).
Finally, since UV light can be more tightly focused than IR, UV lasers support
complex, high-resolution marks such as 2D barcodes. Given all these advantages, why haven't UV lasers been widely employed in medical marking applications in the past? The simple answer was cost. But, over the past decade, companies such as Coherent have made substantial improvements in UV laser lifetime, reliability, and output power. These have been achieved through improvements in laser design, materials, and the implementation of stringent cleanroom procedures during production. Also, automated assembly methods and economies of scale as sales volumes have increased have helped to reduce UV laser price by a factor of nearly five over this period. Marking results The Coherent applications laboratory (Luebeck, Germany) has used a 355nm DPSS laser (MATRIX 355) to mark several different materials that are representative of those used in medical applications. Some of the most relevant of those results are presented here |
 |
| High-density polyethylene
(HDPE) is a plastic widely employed for packaging pharmaceuticals and food, as
well as to make water bottles. Ink marks on HDPE can be removed with solvents,
allowing the product to be relabeled after packaging. Also, the ink is a
possible source of contamination. In this test, the laser was used to mark 2D
barcodes on the curved surface of a pill bottle (FIGURE 1). The 355 nm laser,
working with a focused spot size of 30μm, was scanned over the part surface
using a galvanometer mirror system. In this configuration, high-contrast, 8 ×
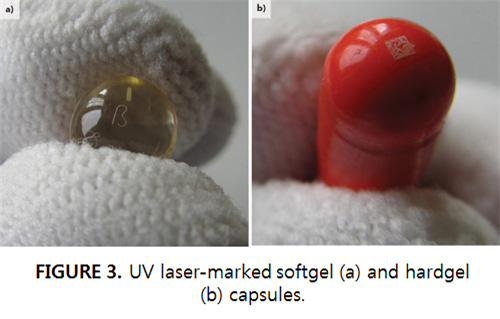
8mm barcode patterns were generated in 2s. The MATRIX 355 is particularly advantageous for producing this type of mark on HDPE because of its PulseEQ mode, which holds pulse energy at a constant value, even as repetition rate is varied. This is not the case with conventional Q-switched lasers. The advantage of changing repetition rate is that it enables the color, darkness, and pulse overlap of the mark to be altered on the fly without affecting other scan parameters. This makes it easier to stay within the marking process window. Another plastic used extensively in the medical field is silicone rubber, which can appear either transparent or white. A common application for this is tubing to connect an intravenous infusion to a patient, or for intubation. Typically, the hose must be marked with its diameter dimension and its storage date (by law, these must be used within three years of manufacture). In this case, the laser was focused on the inside surface of the tube (FIGURE 2) so that marking produced absolutely no change in the texture of the outside surface (which is what comes into contact with the patient). Test marking on various types of softgel and hardgel capsules was performed to determine the maximum achievable marking speed (FIGURE 3). For 1.5mm-high characters on softgel capsules, the highest speed was < 0.024s/character. Mark legibility was excellent in all cases. For hardgel capsules, a 2D barcode measuring 1 × 1mm could be reproduced in < 0.2s. In contrast, ink marking requires a drying time of 1-2s before the pill can be handled without smearing the mark. A type of gelatin is also used to make blister packages for some medical products (FIGURE 4). The goal in this case was to produce a legible mark with a maximum penetration depth of 30% of the thickness (specifically, 0.17mm of the 0.58mm total thickness). The laser pulse energy was 100μJ and the scan speed was 1.3m/s. The laser was purposefully defocused at the work surface to produce a character line width of 160μm. The color change marks show good contrast with no material ablation. Conclusion Testing at the Coherent applications laboratory shows the UV DPSS laser to be an effective source for rapidly producing legible, high-resolution marks on medical devices and pharmaceutical products. These permanent marks compare favorably with printed marks. The UV laser is also advantageous over longer-wavelength lasers since it is usable with a wider range of materials, including plastics and papers, which might not be compatible with a thermal process. |
| 출처:http://www.industrial-lasers.com/articles/print/volume-30/issue-6/features/precision-laser-marking-for-medical-applications.html |
이전글 레이저 관련 국내외 행사
